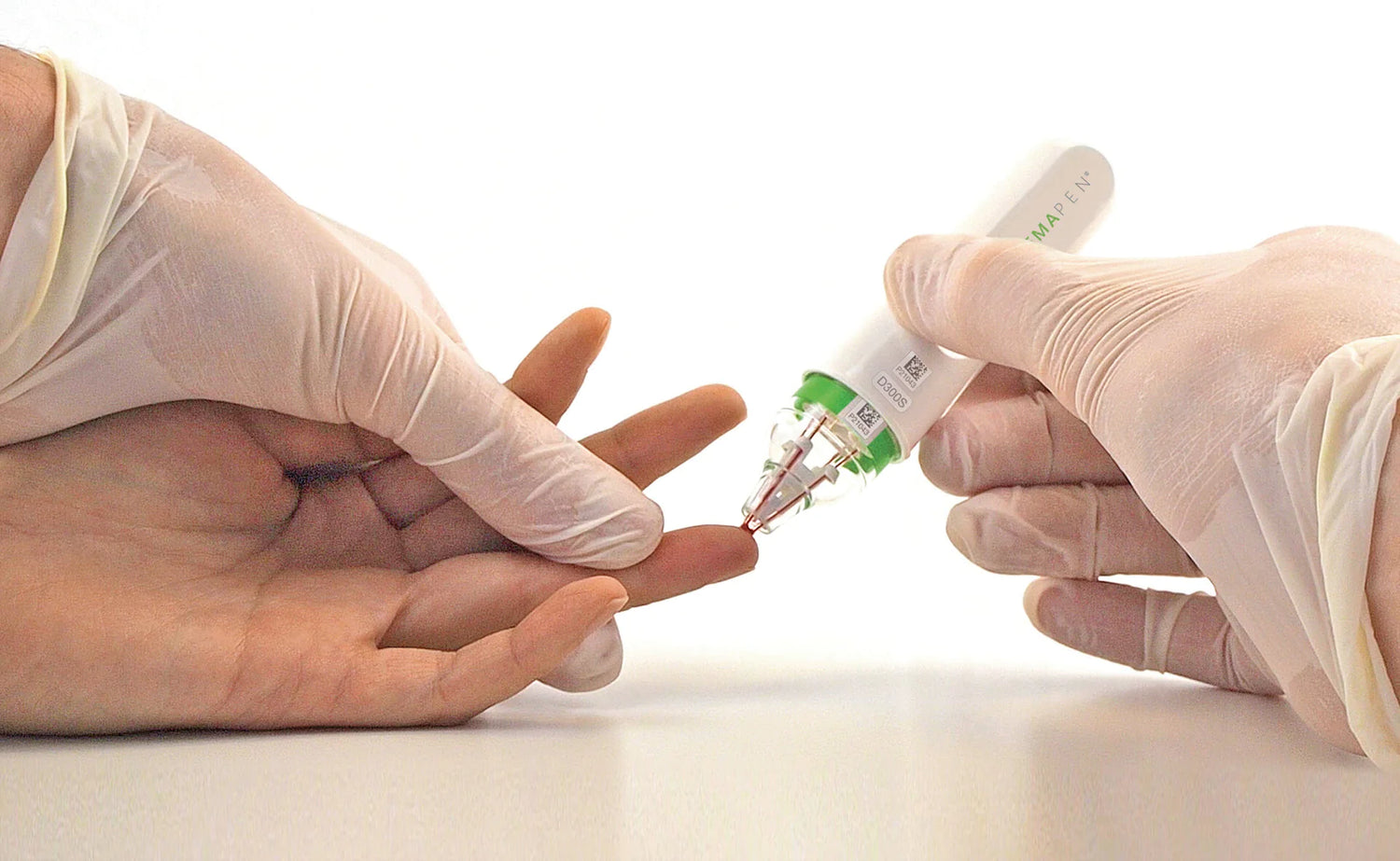
Trajan Scientific and Medical (Trajan) has listed its hemaPEN® blood microsampling device with the U.S. Food and Drug Administration (US FDA) as Class I for therapeutic and in vitro diagnostic (IVD) use.
This follows the hemaPEN’s recent registration in May for supply in the EU and UK as a General IVD; as well as inclusion in the TGA’s ARTG (Australian Register of Therapeutic Goods) in February as a Class I IVD.
This enables hemaPEN to be used to collect blood microsamples for clinical trials, diagnostics and applications to monitor health.
hemaPEN provides a convenient sampling procedure for collection and storage of four dried blood spot (DBS) samples. Unlike conventional DBS sampling tools, hemaPEN enables collection of an accurate and precise fixed micro-volume and is designed to maintain sample integrity for quantitative analysis. An easy-to-use sophisticated microsampling tool in the hands of non-analysts.
Remote microsampling facilitates more flexible blood sampling compared to traditional phlebotomy (blood draw) for scientific and clinical communities, for rapid R&D and implementation of new healthcare solutions for the public.
Trajan believes in science that benefits people – creating portable and affordable measurement solutions, enabling accurate results to inform preventative healthcare.
Visit www.hemapen.com to purchase or learn more about hemaPEN, and to sign up for updates.
To learn more about Trajan’s microsampling technologies and capabilities visit www.trajanscimed.com/microsampling.
 Download
Download
Press release [PDF]
Related news
The third leg in at-home healthcare – microsampling
CE mark for Trajan’s hemaPEN blood microsampling device, now available for diagnostic use across EU and UK
Trajan’s hemaPEN included on TGA’s ARTG as first blood microsampling device for use in Australia
Pens and prospering as a manufacturer
More information
www.hemapen.com
Media contact information
Trajan Scientific and Medical
Tel: +44 (0) 7703 828 309
media@trajanscimed.com